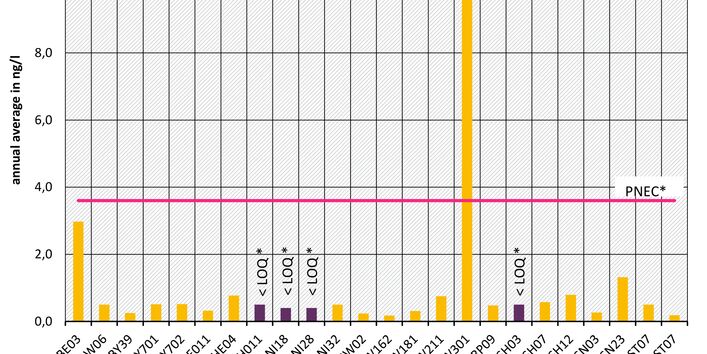
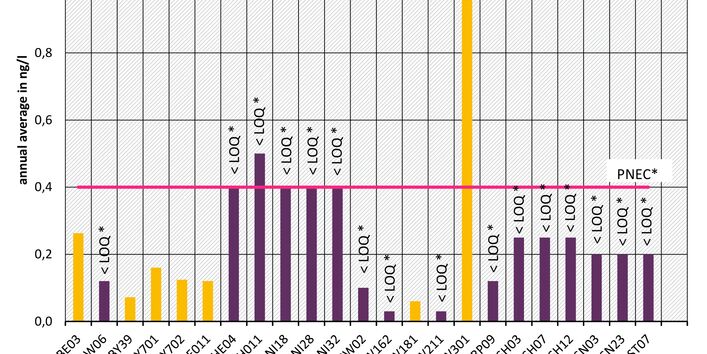
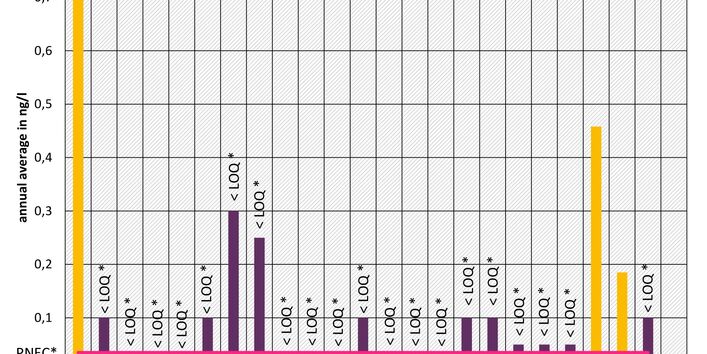
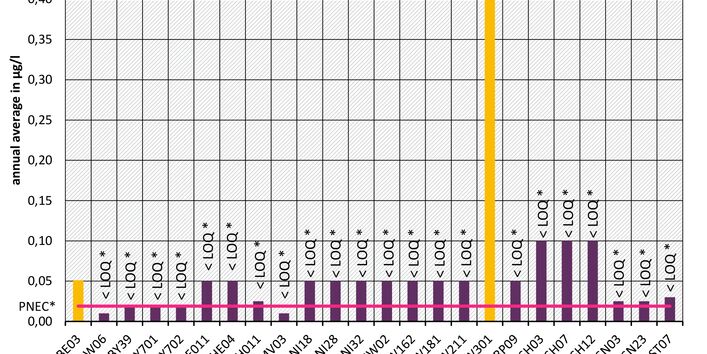
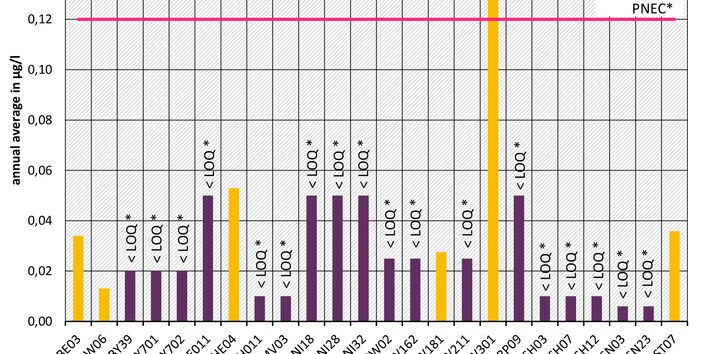
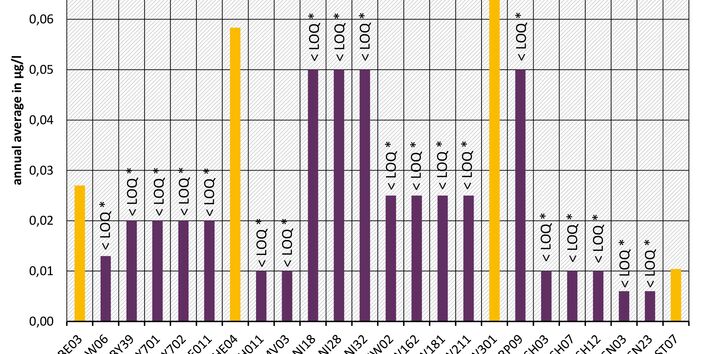
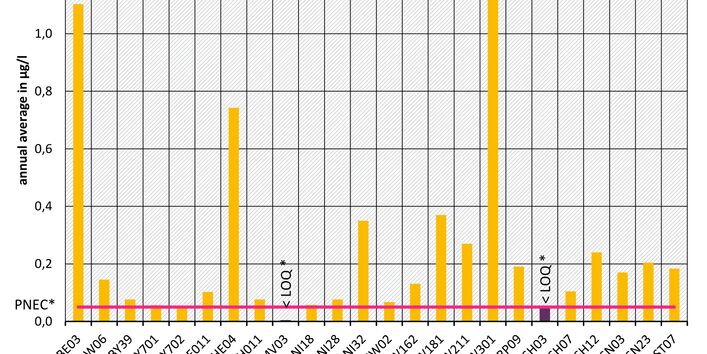
The active pharmaceutical ingredients (API) can also accumulate in rivers in concentrations that are harmful to aquatic biotic communities.
API enter surface waters from the discharges of sewage treatment plants or through their use in livestock production. Therefore the German federal states and the Federal Institute of Hydrology have been measuring select active pharmaceutical ingredients in surface waters for a number of years.
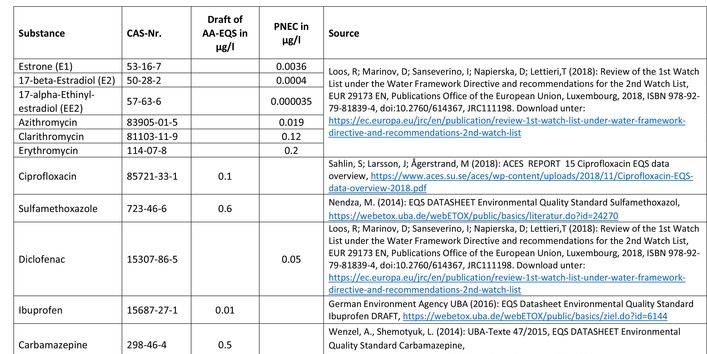
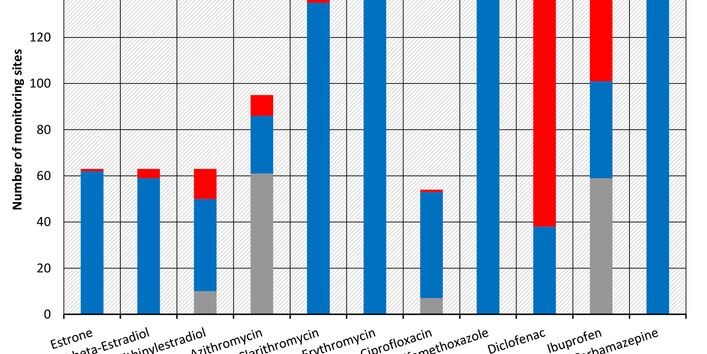
Currently no environmental quality standards have been determined for API either at European or national level. However, monitoring programmes have been initiated at both levels which include measurements of API. A summary of all results 2016 - 2018 in Germany can be found at the end of this article.